
R&D Activity
The R&D laboratory, with over 30 years of well-established and documented academic research experience in the pharmaceutical technology field, is the beating heart of the company and it is able to guarantee innovation and a level of excellence in research.
The main R&D activities concern the design and development of new carrier systems for:
» the modified and controlled release of drugs
» the stabilization of photodegradable or oxidation-sensitive active ingredients
» the increasing of the bioavailability of drugs
» the passive and active targeting of drugs and molecules
» the masking of the bad taste of some active ingredients
The matrices used for the vehiculation/intercalation of active ingredients and molecules of pharmaceutical interest are constituted by synthetic clays and biocompatible inorganic solids. These systems are able to release in a controlled manner high quantities of active ingredient (up to 50% by weight).
The delivery of drugs in inorganic vectors makes it possible to improve the solubility/bioavailability of poorly soluble active ingredients, to control their release in sustained and/or prolonged form, to decrease the toxicity and the side effects of drugs and to realize effective systems for the targeted therapy of different diseases.
The high specific surface area of the inorganic matrices allows the vehiculation of different functional groups such as diagnostic, therapeutic and directional agents. This versatility makes it possible to create multifunctional systems capable of recognizing the target site where the pathology is occurring and at the same time controlling, treating and monitoring the response of the pharmacological treatment.
With the use of nanotechnologies, P&T carrier systems can be easily dispersed/encapsulated in many polymers, forming nanocomposite materials whose properties are greatly enhanced thanks to the inorganic matrix-drug synergistic action.
Polymeric nanocomposite systems can be used for the production of bioactive scaffolds for tissue regeneration (project-fast.eu/en/home), transdermal adhesives and periodontal prostheses.
Our research team employs cutting-edge technologies for the synthesis and modification of clays and biocompatible lamellar solids, ensuring high-quality products, scalability and reproducibility of processes. The production is carried out using green and eco-sustainable methods, involving only water as a solvent and raw materials with high chemical and microbiological purity.
The products are finely chemically-physically characterized using analytical techniques and cutting-edge instrumentation as:
» Structural and phase analysis with X-Ray Diffraction spectroscopy (XRD)
» Thermal analysis by thermogravimetry (TGA), differential thermal analysis (DTA), differential scanning calorimetry (DSC)
» Chemical Composition with Inductively Coupled Plasma Atomic Emission Spectroscopy (ICP/AES) and HPLC Chromatography
» Morphological analysis by Field Emission Scanning Electron Microscopy (FE-SEM)
» Granulometric analysis and dimensional particle size distribution by laser diffraction size analyzer (DLS)
and where appropriate:
» Infrared Spectroscopy (FT-IR)
» UV-visible spectroscopy (UV-vis)
» Measurement of surface area and porosity by adsorption/desorption of gas using the BET method
» Computational molecular modeling with HyperChem software
The R&D department has a formulating laboratory for the study of application of raw materials produced, the development of new projects and the realization of prototypes.
Particular attention is focused on the study of the stability and release of active ingredients carrying out specific tests in accordance with the standards and guidelines of the Official Pharmacopoeia.
Competence and creativity, along with ongoing research, offer to the customer on demand innovative solutions able to anticipate market trends and evolutions.
-
To know more about claysOpen or Close
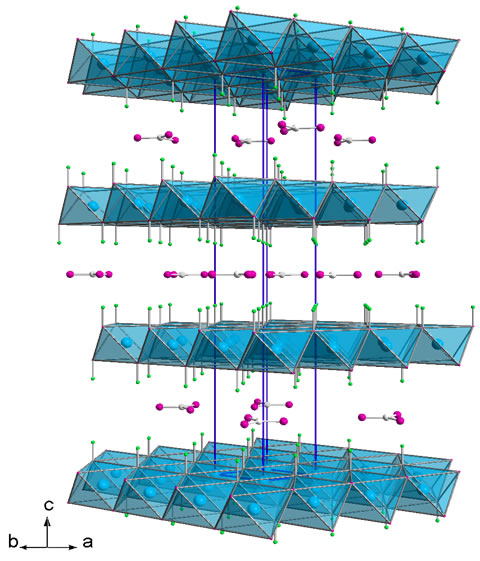
Clay used in the field of cosmetics and health care belongs to the class of hydrotalcites, also known as layered double hydroxides or anionic clays.
Hydrotalcite is a natural mineral having a chemical formula [M(II)1-x M(III)x (OH)2]+x (A-n)x/n · mH2O, the name derives from its resemblance to talc and affinity for water.Its structure is lamellar and consists of stacking layers of mixed hydroxides of magnesium and aluminium having an excess of positive charge, which is counterbalanced by the presence of carbonate anions in the interlayered region.
This natural mineral is very rare and was discovered for the first time in 1842 in a magnesium-serpentine quarry in Snarum, Modum, Buskerud, Norway. Generally, hydrotalcite originates from an alteration of serpentinite and therefore can be traced in combination with minerals such as serpentine, dolomite and hematite.
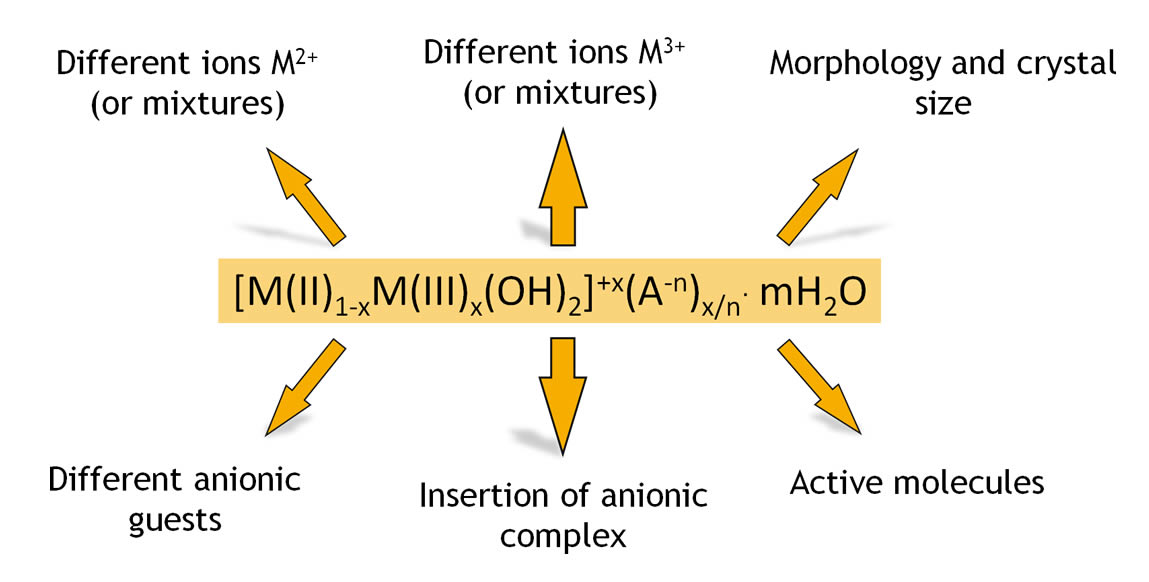
Hydrotalcites constitute a class of lamellar clays with the same structure of the natural mineral and having general formula:
M(II)1-xM(III)x(OH)2(An-)x/n * mH2O where M(II) is a bivalent metal (i.e.. Mg o Zn); M(III) is a trivalent metal (i.e.. Al); An- is an anion with anionic charge n and m is the numbers of moles of water for each mole of clay.
Hydrotalcites, similarly to the natural mineral, have an excess of positive charge in the lamellae that are balanced by the presence of exchangeable anions in the interlayered region.
P&T clays are synthesized reproducing the structure and characteristics of the natural mineral, but guaranteeing:
» high chemical and microbiological purity
» controlled and reproducible chemical composition
» granulometry, dimensional particle distribution and strictly controlled morphologyVariables, characteristics and morphologies
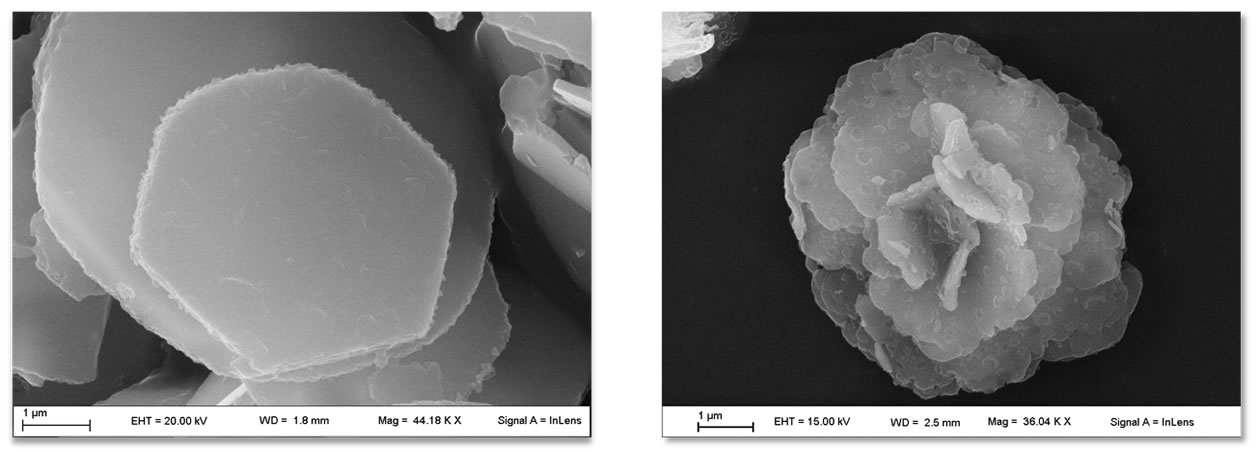
The main characteristic of the hydrotalcites is the ability to exert ion exchange reactions to encapsulate ingredients and APIs in anionic form. Such reactions are carried out by means of the intercalation technology that brings to an active loading up to 50% by weight.
For certain dermocosmetic applications, where metals such as aluminium are not desired (although Al in hydrotalcite is bound and unavailable), Prolabin & Tefarm offers aluminium-free lamellar clays with chemical-physical properties similar to hydrotalcites.
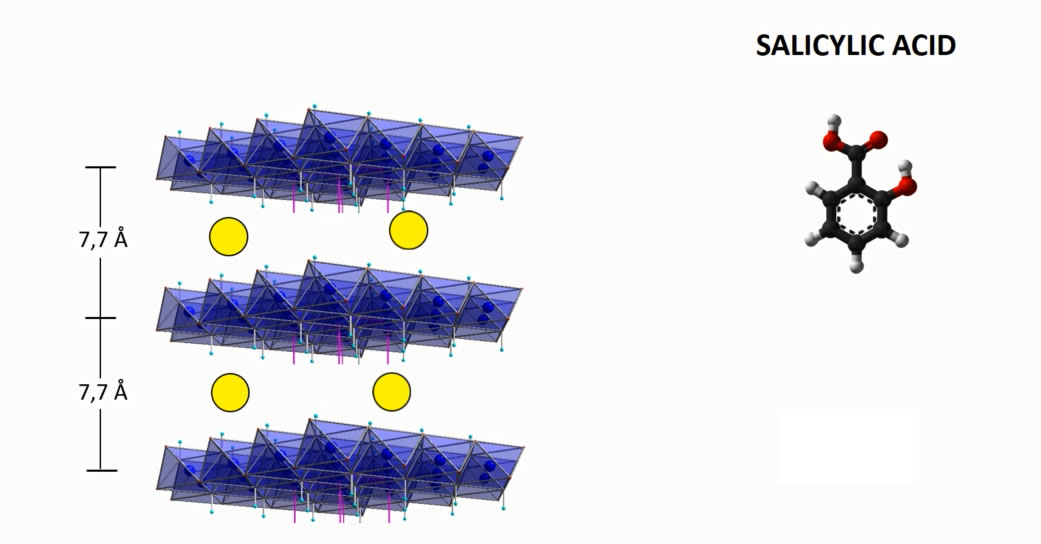
INTERCALATION MECHANISM
Solutions on demand
Prolabin & Tefarm over years has acquired a consolidated and documented know-how on drug delivery strategies and the implementation of modified release active principles. The technologies used are based on the intercalation and/or inclusion of the drug into biocompatible inorganic matrices, which allow the loading of high amounts of active ingredient within the layers or cavities of the inorganic solid and their release under appropriate conditions through an ion exchange mechanism.
Such inorganic hybrid systems allow the development of controlled-release formulations, designing and realizing ad hoc pharmaceutical specialties with the desired release kinetics.
Vehiculating the active substance in inorganic matrices protects the drug from possible degradation due to exposure to light (photodegradation), oxygen (oxidation) and moisture (hydrolysis), greatly increasing the efficacy and safety of the API.
If your company is interested in using an active substance with problems of stability or solubility, if you need to increase the efficacy of the drug in terms of bioavailability or to modify its release in a controlled manner from a particular pharmaceutical formulation, contact us and ask for your personalized technology solution.
Prolabin & Tefarm’s R&D Laboratory is specialized in the design and production of new organic/inorganic hybrids consisting of active principles in biocompatible inorganic solids and can offer you a solution in line with the desired technical characteristics and specific production needs.
The R&D department will evaluate the feasibility of the project and select the best inorganic support able to vehiculate the drug of interest, realize the industrial prototypes and support you in formulating development.
Once the prototype is validated and tested, Prolabin & Tefarm will proceed to the industrial scale-up of the product and will be able to provide the production protocol or directly the new drug delivery system in the desired quantities.
CONTACT OUR R&D LABORATORY AND REQUEST YOUR PERSONALIZED TECHNOLOGICAL SOLUTION
